RHS Academy on Green Issues: Ocean Acidification & Its NY Impact
Recently, students at The Academy at Rye High School, a project based learning program, looked at a variety of environmental issues. MyRye.com will bring you a few of these projects in the run up to Earth Day on Saturday, April 22nd.
Today, junior Jack Acciavatti looks at ocean acidification and its impact on New York.
By RHS junior Jack Acciavatti
Introduction to Ocean acidification
Ocean acidification is a process in which seawater becomes more acidic over time due to the excess carbon dioxide (CO2) it absorbs from the atmosphere. Ocean acidification is a direct result of burning fossil fuels, and at least one-quarter of fossil fuels are dissolved into the ocean.
As CO2 dissolves in water, it reacts with water (H2O) to become carbonic acid (H2CO3). The Hydrogen ions in this compound make water more acidic, lowering the pH of water. This can have a very negative impact, hurting many different shell-forming animals such as oysters, crabs, corals, and urchins. Ocean acidification can reduce the amount of carbonate in water, making it more difficult for these animals to form shells and skeletons. It can also break down existing shells.
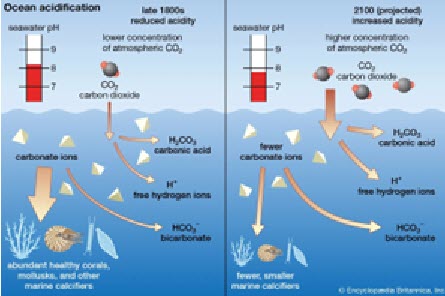
Economic Dependence
As ocean acidification can break down the shells of consumer-based sea animals such as oysters, clams, and scallops, economic problems could occur. For example, New York ranks 6th in financial dependence on shelled mollusks out of all states. This is because of the Long Island Sound, a hotspot for fishing and farming.
According to the New York State Department of Environmental Conservation, shellfish harvests in the Long Island region have contributed over $10 million annually in reported revenue to New York’s local economy. If ocean acidification worsens, New York could potentially lose millions of dollars in shellfish sales.
Along with the danger to animal shells and skeletons, Murat Belivermiş, a scientist at Istanbul University’s radioecology laboratory, discovered that ocean acidification could pose a risk to animals as well as humans with the absorption of certain metals. Lauren Gill reports, “Using radiotracers, Belivermiş and his colleagues discovered that, when exposed to slightly acidified seawater conditions, clams absorbed twice as much cobalt than they would under balanced control conditions, while other marine organisms, such as oysters, have shown a higher level of resilience. This reveals that ocean acidification not only poses a risk to the clams themselves, but also to the people who eat them; cobalt is a heavy metal needed by the human body in minute quantities, but is toxic at elevated concentrations.”
While Belivermis did this test in Turkey, it is also very relevant to New York. This rise in cobalt seen in the trial could have great dangers to New York’s shellfish sales as animals like clams are infested with excess minerals and metals, not only damaging the animals but also posing a health risk for anyone wanting to eat them. This could cause significant harm to New York seafood markets.
Ocean Vulnerability
New York has had an issue with algae blooms over the years, which can also lead to increases in ocean acidification. Along many areas of New York, leaky sewage systems have dumped immoderate amounts of nutrients such as Nitrogen into waterways, spurring excess algae growth. When the algae die and decompose, they release excess carbon that can lead to high acidity levels. This extra carbon is not only bad for animals but swimmers alike. Acidic waters can be damaging to swimmers as they can sting eyes and lead to dry skin and hair, which can also cause itching.
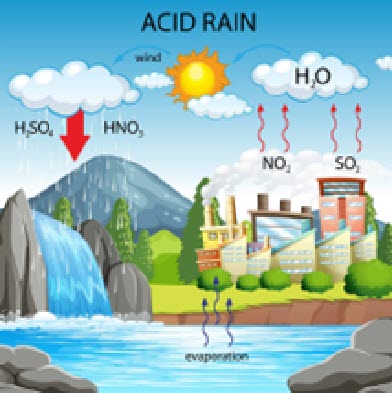
Acid Rain
Acid rain is rain that is unusually acidic and has a pH between 4-5 on average. Normal drinking water has an average pH of between 6.5- 8.5. Acid rain occurs most commonly in the North Eastern area of the U.S., as this area is highly industrialized and home to many cities.
Similar to Ocean acidification, Acid Rain occurs when chemicals are released into the atmosphere, namely sulfur dioxide (SO2) and nitrogen oxides (NOX). These chemicals then can mix and react with water vapor and oxygen, leading to acids that can easily dissolve into particles of water. Acid rain is mainly the product of the burning of fossil fuels. Still, the exhaust from motorized vehicles such as cars and trucks can also release said chemicals. Acid rain relates to ocean acidification as this rain can lower the Oceans pH as the rain is very acidic.
“Acid rain isn’t just a problem of the land; it’s also affecting the ocean,’ said Scott Doney, lead author of the study and a senior scientist in the Department of Marine Chemistry and Geochemistry at the Woods Hole Oceanographic Institution (WHOI). “That effect is most pronounced near the coasts, which are already some of the most heavily affected and vulnerable parts of the ocean due to pollution, overfishing, and climate change.” Doney’s words are very relevant to Rye, as Rye is located on the coast outside a major city. Even though acid rain is fortunately on the decline in America, it still occurs, and can cause great damage to our town and Long Island Sound. One simple way to prevent acid rain is driving less.
Stopping Ocean Acidification
Ocean acidification is a direct result of carbon emissions and the burning of fossil fuels. Lowering your carbon footprint can impact ocean acidification and other issues that come with carbon emissions. Listed below are several ways you can help:
- Choose local and organic food sources
- Reduce food waste
- Don’t buy fast fashion
- Turn lights off when not in use
- Drive less
Jack Acciavatti is a junior in the Academy at Rye High School. He enjoys playing sports, such as football and rugby. He also enjoys baking, and spending time with friends and family.
Further information:
https://you.stonybrook.edu/oceanacidification/ https://ocean.si.edu/ocean-life/invertebrates/ocean-acidification https://ocean.si.edu/ocean-life/invertebrates/ocean-acidification
https://www.eea.europa.eu/data-and-maps/indicators/ocean-acidification-1/assessment https://www.scientificamerican.com/article/ocean-acidification-threatens-the-u-s-economy/
https://www.iaea.org/newscenter/news/jeopardy-at-sea-what-atoms-in-clams-tell-us-about-ocean-acidifica tion
https://www.sciencedaily.com/releases/2007/09/070907175147.htm#:~:text=The%20release%20of%20sulfur
%20and,by%20atmospheric%20and%20marine%20chemists





